'I am relieved': Quebec to cover ALS medication after repeated calls from patients
A drug known to treat amyotrophic lateral sclerosis, more commonly known as ALS, will be covered by Quebec’s drug insurance plan – a change doctors and those living with ALS have been demanding for years.
ALS, also called Lou Gehrig’s Disease, is a rare neurological condition affecting the nerve cells responsible for voluntary muscle movement, such as chewing, walking and talking. It’s progressive, meaning those symptoms tend to progress over time, until the brain is no longer able to initiate voluntary movement.
There are no cures for ALS, but there are treatments to slow its progression. One of them is Albrioza, typically taken dissolved in water or ingested through a feeding tube.
Amanda Tam has been living with ALS for about two years – she says she first started feeling off just days before her 21st birthday.
"A lot of twitching, grip strength was very strange, very weak. Doing exercise was very hard," she told CTV back in March.
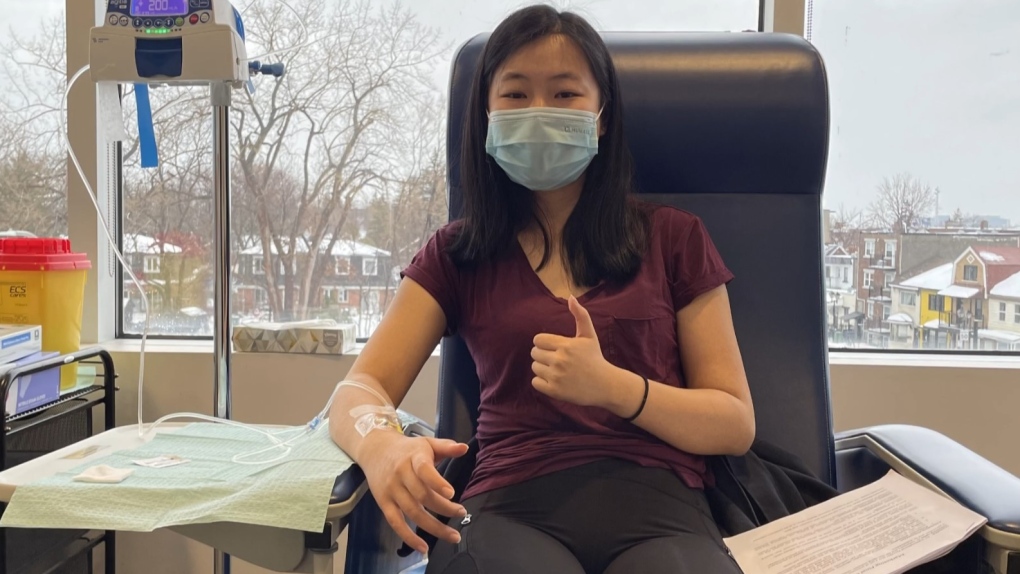 Amanda Tam found out she has ALS four days before her 21st birthday.
Amanda Tam found out she has ALS four days before her 21st birthday.
Since her diagnosis, she has shared her experience on social media to show others what being a young person with ALS is like. Her videos, posted on TikTok, have garnered tens of thousands of views.
She had also been taking Albrioza, which had only been approved by Health Canada months earlier. She got access to it before others through private insurance. She was vocal about her success with the drug, and one of many calling on the province to cover it so that others could benefit from it too.
Even for her, access to Albrioza was precarious. The private insurance would only cover it while she was a student, and a prescription for the drug costs around $94,000 per year.
That was her reason for going public with her story back in April. Since then, she’s graduated from university, and pharmaceutical company Amylyx has maintained her supply, even without insurance.
And faced with Thursday’s news that other patients would have it covered, she said it gave her hope.
“I am pleased that the government is moving forward and granting patients with a terminal illness a medication they are entitled to have,” she told CTV in a statement, adding she hopes Quebec’s approval is just a first step.
“It is unfortunate that most patients with ALS still cannot access it, and it is frustrating since every person battling this disease should be provided with the medication they are intended to take,” she said.
“I am relieved, that's the most overwhelming emotion,” said Angela Genge, director of the ALS program at the Montreal Neurology Clinic. “Our patients in Quebec have been ambassadors for this medication.”
Tam has been one of those advocates and said that she hopes more people are able to get the medication covered in the future.
“Unfortunately, they are not covering those who are past their 18 months post-diagnosis,” she told CTV. “I am glad they are finally here to support those in need of the medication, but it is unfair to patients who have been suffering from the disease and still can’t access (it).”
Genge said its limited availability is a symptom of its trial process, and not necessarily final.
“When we're doing a clinical trial, what we're trying to do … is to determine whether a drug is truly effective,” she said. “In order to do that, you need the group on the drug and on the placebo to be as homogeneous as possible.”
She said that patients who don’t meet the 18-month criteria could still get the drug covered, they’d just have to go through different channels, which is something her clinic does regularly.
Until more people are covered, she said, “we’re going to keep on trying.”
CTVNews.ca Top Stories

Trudeau talks border, trade in surprise dinner with Trump at Mar-a-Lago
Prime Minister Justin Trudeau discussed border security and trade during a surprise dinner with U.S.-president elect Donald Trump at Mar-a-Lago in West Palm Beach, Fla. on Friday evening, according to senior government sources.
Man who died trying to help stranded motorist identified as Khalid Farooq, father of 5
The man who lost his life trying to help a stranded motorist Wednesday has been identified as Khalid Farooq.
W5 Investigates 'I never took part in beheadings': Canadian ISIS sniper has warning about future of terror group
An admitted Canadian ISIS sniper held in one of northeast Syria’s highest-security prisons has issued a stark warning about the potential resurgence of the terror group.
Are scented candles bad for you? What the science says
Concerns about the safety of candles are rooted in the chemical reactions that occur when you burn them, as well as in the artificial fragrances and colorants that contribute to the various scents you may love.
Premier League trophy in Toronto as Man City visits Liverpool in high-stakes showdown
Manchester City's Premier League title hopes could hang in the balance Sunday when the slumping club visits league-leading Liverpool.The trophy they are both battling for is 5,450 kilometres away — in Toronto.
Why this Toronto man ran so a giant stickman could dance
Colleagues would ask Duncan McCabe if he was training for a marathon, but, really, the 32-year-old accountant was committing multiple hours of his week, for 10 months, to stylistically run on the same few streets in Toronto's west end with absolutely no race in mind. It was all for the sake of creating a seconds-long animation of a dancing stickman for Strava.
It's time for a good movie this holiday season, here's what's new in theatres
This holiday season has a special edition at the theatres with movies "that everyone has been waiting for," says a movie expert from Ottawa.
Poilievre suggests Trudeau is too weak to engage with Trump, Ford won't go there
While federal Conservative Leader Pierre Poilievre has taken aim at Prime Minister Justin Trudeau this week, calling him too 'weak' to engage with U.S. president-elect Donald Trump, Ontario Premier Doug Ford declined to echo the characterization in an exclusive Canadian broadcast interview set to air this Sunday on CTV's Question Period.
Emboldened 'manosphere' accelerates threats and demeaning language toward women after U.S. election
An emboldened “manosphere” has seized on Republican Donald Trump ’s presidential win to justify misogynistic derision and threats online.